Sustainable Rigid Films
Explore our range of SecondLife® MD medical device packaging films, crafted with a focus on sustainability. Utilizing certified recycled content, our films meet the stringent requirements of ISO 11607-1 and ISO 10993-5 regulations. This eco-friendly approach ensures that your medical devices are securely packaged while minimizing environmental impact. Rely on SecondLife® MD for packaging solutions that prioritize both sustainability and regulatory compliance.
SecondLife® MD
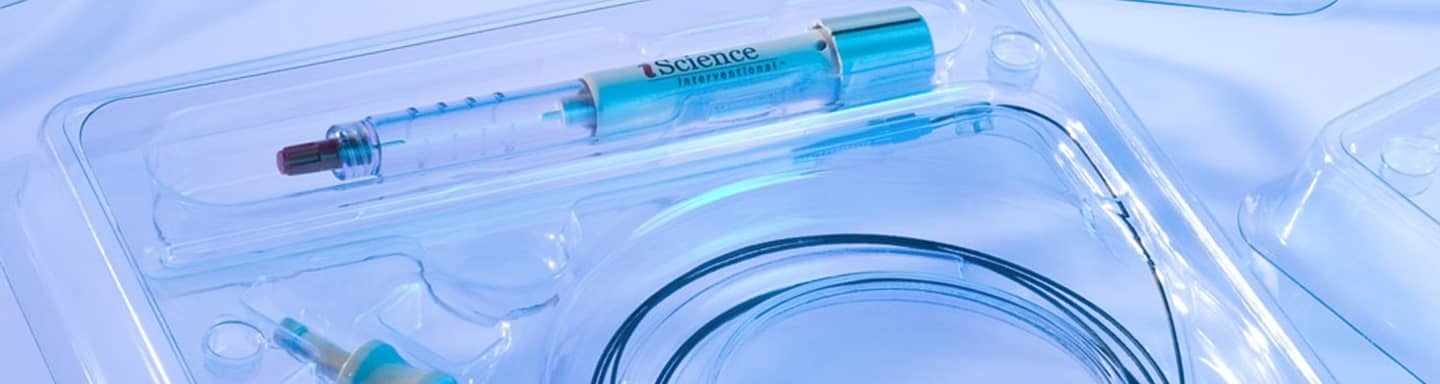
Applications:
- Orthopedic
- Endoscopy
- Neurological
- Cardiovascular
- Surgical
- Wound Care
- Vision
- ENT
Benefits:
- Medical grade PETG with up to 50% certified recycled content*
- Molecularly equivalent to Eastar™ 6763
- Regulatory compliant; ISO 11607 & 10993 and ISCC Plus certified
Polymers:
- PETG
Pentamed® kpNext® MDR1

Applications:
- Orthopedic
- Endoscopy
- Neurological
- Cardiovascular
- Surgical
- Wound Care
- Vision
- ENT
Benefits:
- Best-in-class protection, clarity, and processing performance
- Designed for recyclability in RIC 1 recycling stream
- Manufactured globally under a single, global specification
- Comprehensive regulatory compliance; FDA and EU direct food contact | ISO 11607-1 & 10993-5
Polymers:
- Copolyester Blend







